photons
energy
A photon is energy
Yes, a photon is the smallest, indivisible part of energy
Photons are everywhere
Photons are involved in radio communication, cellular communication, light, and heat transfer. Humans are constantly emitting and absorbing photons
The speed of light
This is the speed of energy transfer in space. The Sun transfers energy to the Earth using photons at the speed of light
Photons diversity
Photons differ in wavelength and transported energy. Just like no two snowflakes are the same, no two photons are identical
Quantum leap
The emission of a photon including the very real quantum leap
Photons source
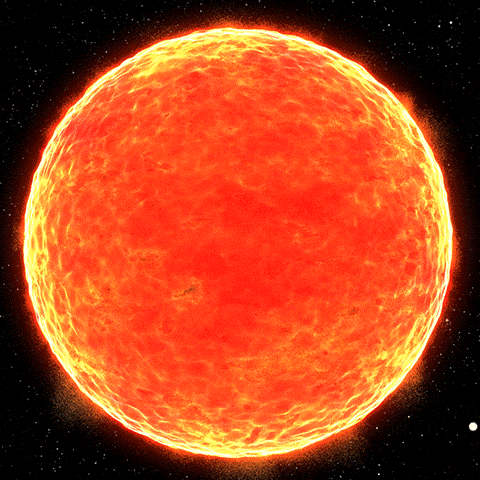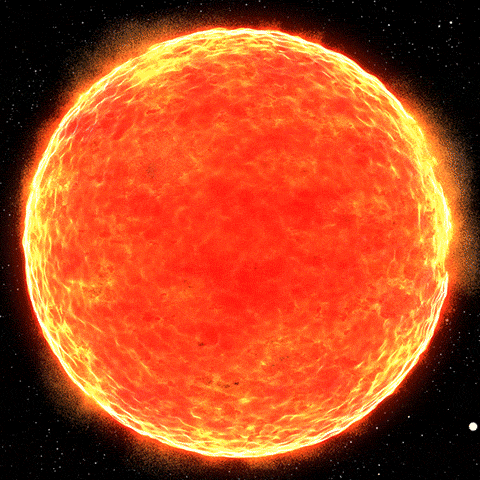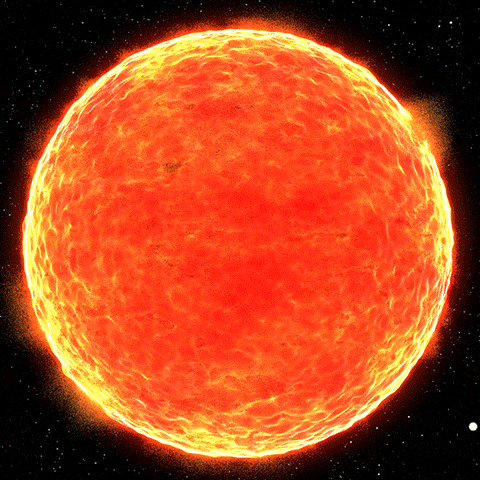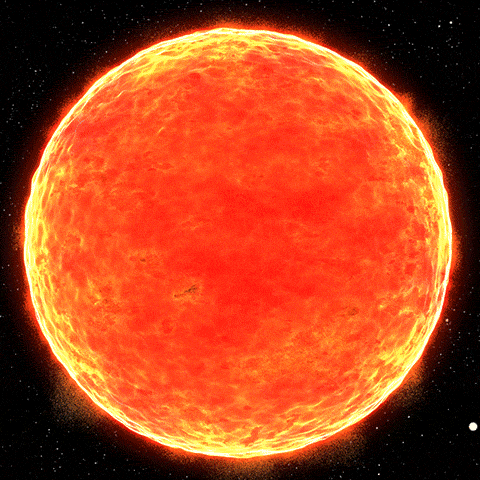
The Sun is a major source of high-energy photons. It’s impossible to simulate the sunlight on Earth
Albert Einstein
Electrons in atoms are located at discrete energy levels. When electrons transition between these levels, photons are absorbed or emitted by the atom
A photon is energy
The photon is something unique and significant. It is a quantum of electromagnetic radiation. A particle that transfers energy from one place to another. When we talk about the energy of the Sun, we talk about photons. They are the actual carriers of energy in our universe.
The photon is an energy carrier. According to the principle of communicating vessels, energy flows to where there is less of it. If a body is heated and the environment is colder, the body emits photons and the environment absorbs them

Photons are a means of transferring excess energy
Photons move at the speed of light in a spiral, and their characteristic is the wavelength of this spiral. This wavelength determines the energy of the photon. The shorter the wavelength, the higher the energy of the photon. Photons with short wavelengths, such as ultraviolet and gamma rays, have the highest energy
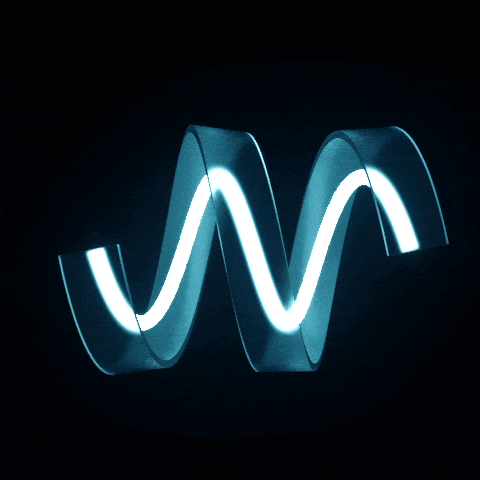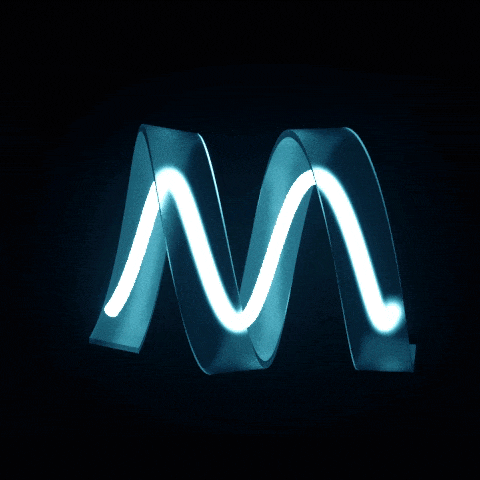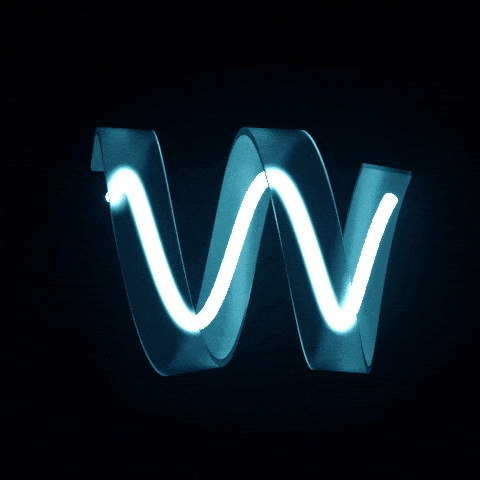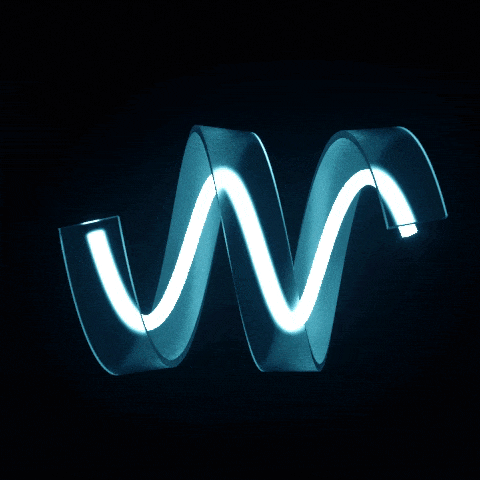
Interaction of photons with other objects
Suppose a photon encounters an obstacle in its path. In that case, it either reflects and continues its flight, or it is absorbed and transfers its energy to the object, heating it. Sometimes, the photon transfers part of its energy, continuing its path but with an increased wavelength. This process is known as impulse transmission. The photon can convey part of its energy to another photon or an elementary particle through this impulse
For a photon, time does not exist. It travels at the speed of light and does not ‘see’ other light. In fact, light from a distant galaxy or from the Sun arrives instantaneously from the photon’s perspective. The medium of propagation ‘slows down’ photons, so in the Earth’s atmosphere, they travel slower than the speed of light, and even slower in water or dense gas

Types of photons
Photons are either visible or invisible. Invisible high-energy photons (with very short wavelengths) include, for example, x-rays and gamma rays.The visible spectrum ranges from 380 to 780 nanometers. We also do not see photons with wavelengths greater than 800 nanometers; this radiation is called infrared and is a carrier of thermal energy. In a cool environment, a person constantly emits photons, but low-energy ones, with wavelengths much greater than 800 nm. You can notice this radiation using a thermal imager. Radiation with longer wavelengths is used by humanity everywhere, for example, in cellular or radio communications
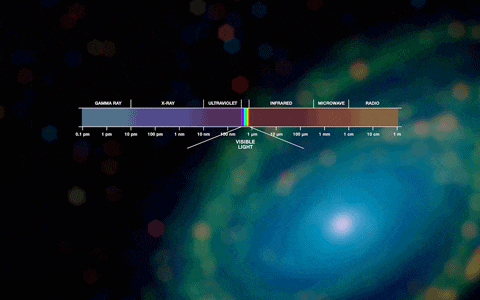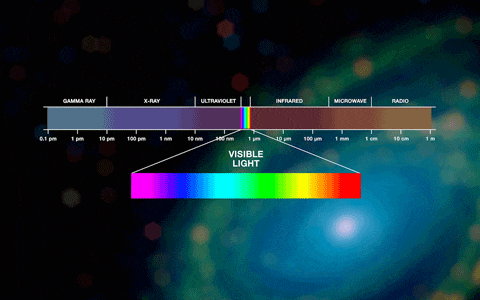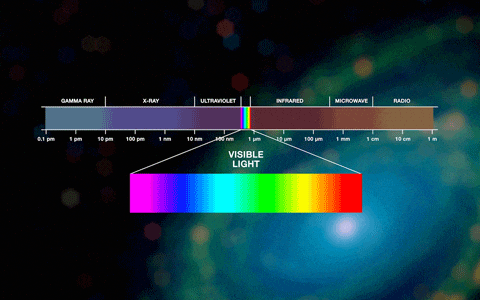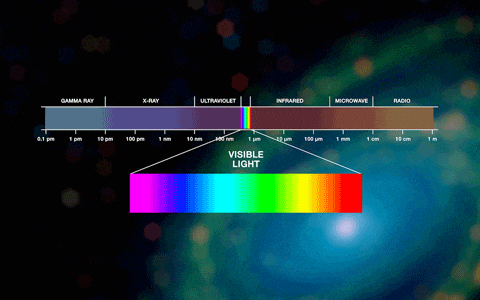
The blackest body
Photons are best absorbed by black bodies (for example, coal). The blackest body in the solar system is the Sun. Since it burns, it emits photons. If the Sun were to suddenly go dark, it would start absorbing photons and could eventually collapse into a black hole
The structure of the photon is currently unknown. It is only understood that it occupies a volume equal to the wavelength cubed. Photons are the most common and well-known particles in the universe. There are approximately 20 billion photons for every electron or proton
Virtual photons
Like every particle, the photon has an antiparticle – a virtual photon. Unlike a real photon, a virtual photon does not exist in time. It represents the energy of interaction that particles with mass exchange instantly or at the speed of light. For example, an electron orbiting an atom constantly exchanges virtual photons with a proton (‘back and forth’). In the atom’s nucleus, protons and neutrons are held together thanks to the constant exchange of virtual pi-mesons (pions). Thus, a real photon absorbed by the atom becomes virtual and exists by continuously moving between the electron and the proton, preventing the electron from flying away

A quantum leap
How does a body emit photons? Photons are energy packets that are emitted when the energy of the environment is exceeded. Photons are emitted, or “born” transitioning from virtual to real due to, for example, an increase in temperature or the influence of electricity. Then, the electron in the atom moves to a higher energy level. When it returns, it emits a photon of a particular wavelength. A quantum leap occurs
Photons source
A body can also spontaneously emit photons when another photon passes by. This phenomenon is known as the ‘mirror’ effect, which forms the basis of laser amplifiers. The photon is repeatedly reflected from the mirror surface, each reflection effectively doubling the number of photons and enhancing coherence (aligning photons of the same wavelength). In this way, the mirror strengthens the photons, continually reflecting and amplifying them

Why are photons so important?
This website was created to highlight the influence of photons on people and their actions. Every second, trillions of photons pass through, reflect off, or are absorbed by the human body. This includes rare, relic waves, as well as a variety of other wavelengths. The Sun is the primary source of the extensive range of photons on planet Earth. While there are many studies on the influence of solar photons, more research is needed. Many people neglect the impact of solar photons on humans!

question - answer
Do you have a Question?
The Sun emits photons with varying wavelengths; the shorter the wavelength, the more energy the photon carries. The Sun is the main source of useful photons in the visible and IR ranges
Photons are absorbed by all bodies, but best of all by black bodies. The shape and size of an object determine which wavelengths of photons pass through without being absorbed. For example, if a body is 1 inch wide, wavelengths longer than 1 inch will pass through it. That’s why radio waves pass through small walls
Nothing in our world can disappear. The modern Standard Model of quantum physics has no exact answer, but it is possible that a photon, after transferring energy by heating a body, becomes a virtual photon existing between an electron and a proton. It is then released when the body is heated. This is an important question, and we are studying it
A lot is known about them, but they are mentioned casually in schools. Many mysteries are explained by photons. Now, the strongest minds on the planet are studying these magical particles, including at the Large Hadron Collider
That’s a lot. For every proton, there are at least 20 billion photons! The surface of the Earth receives about 2.5 × 10^26 (that’s 26 zeros!) solar photons per square meter every second